half life formula for first order reaction
The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction. The half-life equation for a first-order reaction is latextextt_frac12fractextln2textklatex.

Chemical Kinetics Class 12 Notes Vidyakul Chemical Kinetics Chemistry Education Chemistry Worksheets
Half-life of First-order Reactions.

. The half-life equation for a second-order reaction is t1 2 1 kA0 t 1 2 1 k A 0. T ½ A o 2k For a first order reaction A products rate kA. Half-life refers to the amount of time it takes for half of a particular sample to react.
For a first-order reaction the half. The rate for this order is rate k A. Half Life Calculator first order reaction - Free Chemistry Widget.
The half-life equation for a first-order reaction is t12ln2k t 1 2 ln 2 k. For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao. Notice how it takes the same amount of time for the concentration to decrease between points.
Not a set value that we can calculate. The Half-life of the Rea ction. And we typically use the concept of half-life to for example determine the age of ancient artifacts or predict when a radioactive sample will be safe to handle.
Half Life Calculator first order reaction Added Dec 9 2011 by ebola3 in Chemistry. Half-life Method of Determining the Order of the Reaction. Half Life period of first order reaction is the time required for 50 percent completion of the reaction and is represented as t 05 ln 2 K or Half Life Period ln 2 Rate constant.
According to the definition of half-life at time t 12 the concentration of the reactant A is one-half of its initial concentration. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2. Lesson 20 Half-life equations for 1st 2nd and 3rd order.
The formula for half-life for a first order reaction is. T12 0693 k. For the first order reaction you can plug the definition of the half life into the concentration-time reaction to obtain a neat relationship.
The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02. Determining a half life. Graphical relations and half lives.
Why is the half-life of a first order reaction constant. The half-life of a reaction is the time required for a reactant to reach one-half its initial concentration or pressure. The half-life of a first-order reaction will thus be equal to.
First order reactions have unique graphs such as the one below. Rearrange the equation so that all concentration dependent terms are collected on one side and all time dependent terms are on the other. Radioactive decay reactions are first-order reactions.
Ln 2 0693 kt12. Taking the natural logarithm of both sides of the equation in order to eliminate e the following equation is obtained. A a t12 ln2 k a a.
T ½ 1 k A o Top. Rate constant is the coefficient of proportionality relating the rate of a chemical reaction at a given temperature to the concentration of reactant or product. The half-life equation for a second-order reaction is latextextt_frac12frac1textktextA_0latex.
Half-Life of a Chemical Reaction. T t 12 and A t ½ A 0. For a zero order reaction A products rate k.
This widget calculates the half life of a reactant in a first order reaction. Converting a half life to a rate constant. The half-life is constant regardless of the concentration of the reactant.
Unlike a first-order reaction in a zero- or second-order reaction the half-life is dependent on the initial concentration ie. Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial value. Radioactive decay reactions are first-order reactions.
The rate law for a first order reaction is A A0e-kt. The half-life equation for a first-order reaction is t1 2 ln2 k t 1 2 l n 2 k. The half-life equation for a zero-order reaction is t1 2 A0 2k t 1 2 A 0 2 k.
An important thing to notice here is that the half-life of a first-order reaction depends exclusively on the rate constant of. In other words the initial concentration of the reactant has no influence on the half-life of the reaction ie. Ln 12A0A0 -kt12.
Thus the half-life of a first-order reaction is equal to 0693k where k denotes the rate constant whose units are s -1. L n 1 2 k t 1 2. For a zero-order reaction the mathematical expression that can be employed to determine the half-life is.
Learn the half life formula here. An equation relating the half-life of a first-order reaction to its rate constant may be derived from its integrated rate law. Half life is a particular phenomenon that takes place every day in various chemical reactions as well as nuclear reactions.
What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions. Integrating from time 0 to time t the first order integrated rate equation is The half life is the time required for the concentration to drop to one-half its original value. General Chemistry I CHEM 1441 1.
Equations for Half Lives. T 1 2 0693 k. For a first-order reaction the half-life is given by.
For a second-order reaction. Notice that the half life does not depend on the reactant concentration. The half-life t 12 of a reaction is th e length of time it t akes for the concentrati on of the reactant to fall to ½.
T 1 2 0693k. Substituting the values in the expression for the rate constant of half-life first-order reaction the.

Neet Chemistry Notes Chemical Kinetics Order Of Reaction 8 Https Www Cbsetuts Com Neet Chemistry Not Chemical Kinetics Chemistry Notes Chemistry Classroom

Chemistry Formula For Class 12 Chapter Chemical Kinetics Chemical Kinetics Chemistry Chemistry Class

Chemical Kinetics And Rate Laws Chemical Kinetics Chemistry Notes Chemistry Worksheets

Rate Of A Chemical Reaction Chemical Kinetics Chemical Kinetics Chemical Reactions Ap Chemistry

Chemical Kinetics And Half Life Online College Chemistry Courses Chemical Kinetics Half Life Physical Chemistry
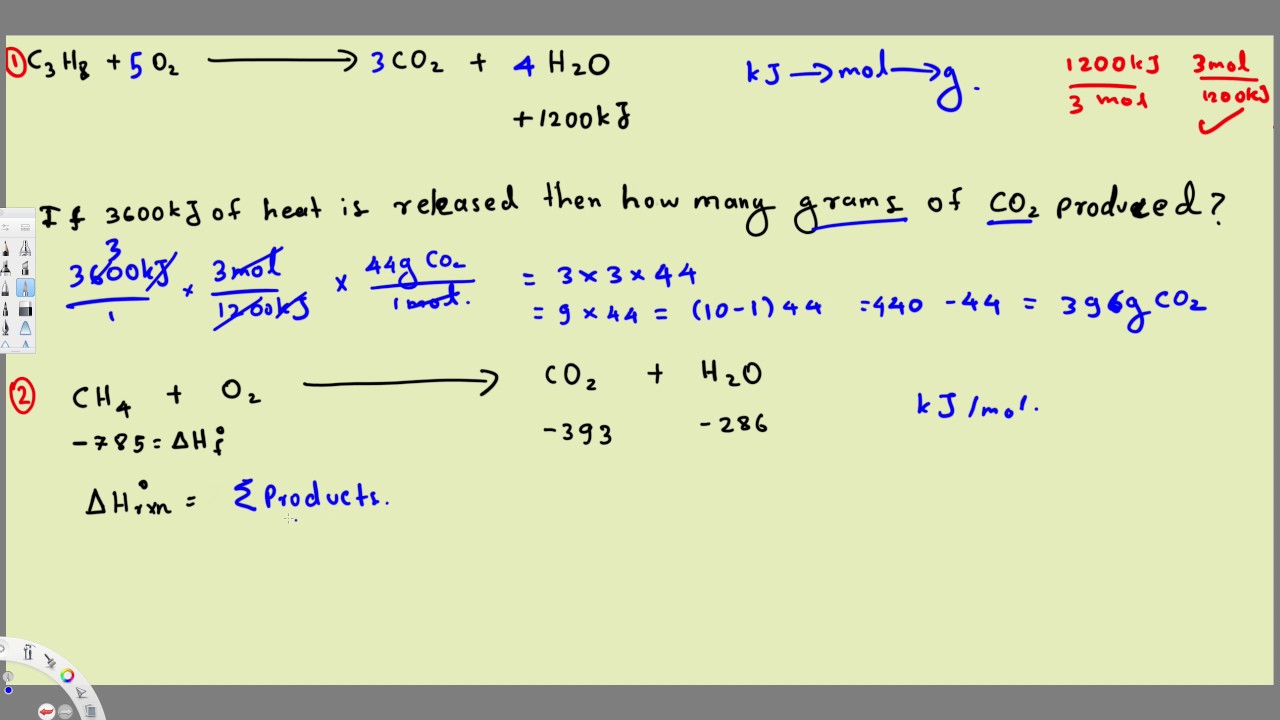
Thermochemistry Equations Formulas Practice Problems Example 2 Equations Chemistry Practice

Chemical Kinetics In 2021 Chemical Kinetics Chemistry Experiments Chemistry Class 12

Half Lives Chemistry Classroom Teaching Chemistry Chemistry Experiments

Neet Chemistry Notes Chemical Kinetics Order Of Reaction 8 Https Www Cbsetuts Com Neet Chemistry Not Chemical Kinetics Chemistry Notes Chemistry Classroom

Van T Hoff Equation Physical Chemistry Chemical Equation Secondary Science

Way To Find Atomic Mass Of Elements Molar Mass Atom Element

Learn Rate Law And Rate Constant For Reactions Of Different Orders Chemicalkinetics Ratelaw Rateconstant L Chemistry Education Physical Chemistry Chemistry

Ncert Solutions For Class 12 Chemistry Chapter 4 Chemical Kinetics Cbse Tuts Chemicalkineticsclass12ncertsolutions Chemical Kinetics Chemistry Solutions

Half Lives Chemistry Lessons Teaching Chemistry Half Life

Learn Rate Law And Rate Constant For Reactions Of Different Orders Chemicalkinetics Ratelaw Rateconstant L Chemistry Education Physical Chemistry Chemistry

A Plot Of Concentration Of Reactant Versus Time Is A Straight Line For A Zero Order Reaction The Half Life Physical Chemistry Ap Chemistry College Chemistry

1 C This Shows The Equation Dealing With Frequency Wavelength And Speed And How To Persuasive Writing Prompts Text Structure Worksheets Chemistry Worksheets

